 |
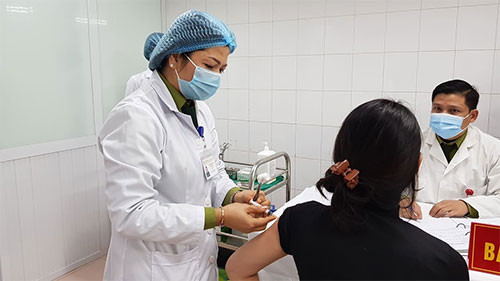
| A volunteer receives the second shot of the Nanocovax vaccine. (Photo: VNA) |
The three, comprising one male and two females aged between 20 and 25, are among the first 20 volunteers given the vaccine on December 17, 2020.
Prof. Do Quyet, Director of the Hanoi-based Vietnam Military Medical University, said the second shot comes 28 days after the first one.
As volunteers haven’t shown abnormal symptoms after getting the first injection, the 25mcg, 50mcg, and 75mcg doses of Nanocovax have been tested on remaining groups of volunteers safely.
So far, all volunteers have been in stable health condition. Some felt pain at the injection site or mild fever, but the symptoms disappeared after 24 hours, Quyet noted.
Assoc. Prof. Chu Van Men, Director of the university’s clinical trial centre, said 50 percent of the first phase of human trials has completed.
The university has received more than 500 applications for Nanocovax trials, but only 51 are eligible for joining the first phase of testing due to strict selection criteria.
Phase I, with the doses of 25mcg, 50mcg, and 75mcg injected, aims to evaluate the vaccine’s safety. Phase II is set to conduct testing on 560 people and begin in February to assess the vaccine’s effectiveness. Meanwhile, the third phase will need at least 1,500 - 3,000 volunteers, including those in other countries.
If everything goes as planned, the first two phases will finish in February and be followed by the third one when half of Phase II is completed.
Vietnam is one of the 40 countries that have conducted human trials of a COVID-19 vaccine.
Nanocovax is developed by the Nanogen Pharmaceutical Biotechnology JSC, basing on recombinant protein technology.
The country also has several other COVID-19 candidate vaccines being developed, by the Institute of Vaccines and Medical Biologicals (IVAC), the Company for Vaccine and Biological Production No.1 (VABIOTECH), and the Centre for Research and Production of Vaccines and Biologicals (POLYVAC).
Source: NDO
















.jpg)





.jpeg)

.jpeg)


.jpeg)


