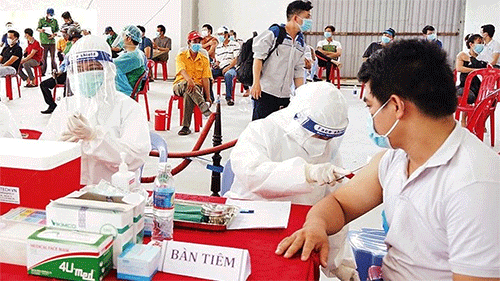
 |
| Vaccination against COVID-19 for workers at Song Than 3 Industrial Park, Thu Dau Mot city, Binh Duong province. (Photo: NDO/Trinh Binh) |
According to the Ministry of Science and Technology (MoST), up to now, many scientific research projects at the state, ministerial and grassroots levels have been carried out to research or transfer and master the technology to produce essential vaccines and important biological products for the care and protection of public health.
It can be affirmed that Vietnam is very successful in absorbing technology transfer, deploying from research and development (R&D) projects to pilot implementation and production on industrial scale.
Facilities that engage in vaccine research and production have research and development units funded by the State and receive technology transfer from some advanced countries at the same time. However, there are not many vaccines using new technologies manufactured in Vietnam due to limited funding. Thus, the country still have to import them.
Since the outbreak of the COVID-19 pandemic, many private enterprises have invested in research and technology transfer to produce COVID-19 vaccines using different types of technology.
Currently, Vietnam has four facilities that engaged in vaccine research and production and an unit that receives technology transfer for COVID-19 vaccine production from abroad. Of these, two are in clinical trials.
Given the complicated developments of the COVID-19 pandemic, the Government, the MoST, the Ministry of Health (MoH) and relevant ministries and branches have focused on supporting research and production activities, as well as building mechanisms to facilitate the development and use of domestically produced vaccine products.
Organisations and enterprises that engaged in research and technology transfer for vaccine production are entitled to preferential policies. For vaccines against the COVID-19 pandemic, units are assisted with the maximum level of 100% of funding for research, clinical trials, trial production, insurance and support for volunteers.
According to the MoH, once Vietnam's Nanocovax COVID-19 vaccine meets the standards and is granted licence, the World Health Organisation (WHO) will consider including it in the COVAX programme. In addition, dozens of countries have signed a memorandum of understanding to request cooperation, technology transfer and distribution of Vietnam's vaccine.
It is projected that each dose of Nanocovax, a Vietnam’s homegrown COVID-19 vaccine cost VND120,000 (US$5.3) and the price will not be changed.
In response to a proposal from the Nanogen Pharmaceutical Biotechnology Joint Stock Company on dispatching a task force to assist it in handling related administrative procedures, Minister of Health Nguyen Thanh Long said that he would streamline administrative procedures, only focusing on resolving professional issues so that the Nanocovax vaccine can be licensed soon.
















.jpg)





.jpeg)

.jpeg)


.jpeg)


