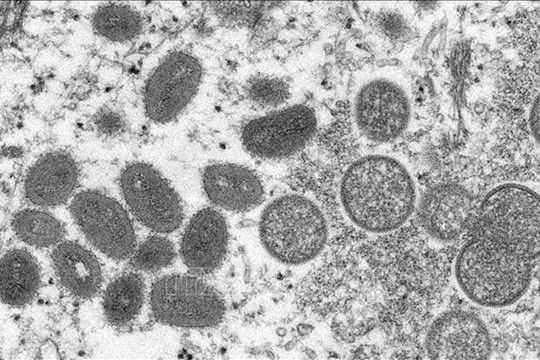
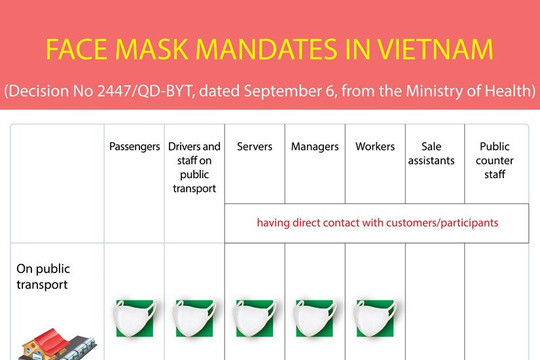
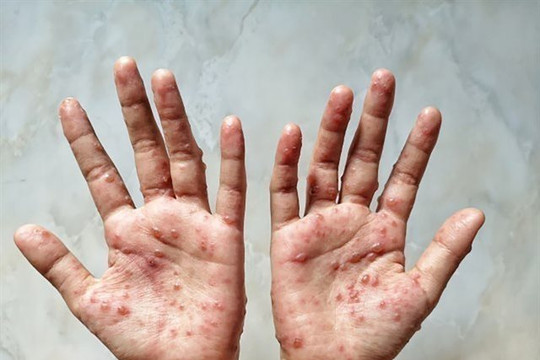
 |
| A laboratory of Arcturus Therapeutics (Photo courtesy of the company) |
With a capacity to manufacture 200 million doses per year, Vingroup is projected to produce the first batches of vaccine by early 2022.
Arcturus Therapeutics is a leading clinical-stage messenger RNA medicines company focusing on the discovery, development and commercialisation of therapeutics for rare diseases and vaccines.
Under the agreement, the US firm will grant permission for Vingroup’s affiliate VinBioCare to produce COVID-19 vaccine named VBC-COV19-154, which is effective against new variants of the coronavirus such as Delta and Alpha.
The Vietnamese company is also approved to manufacture other COVID-19 vaccine types of Arcturus like ARCT-021.
The transfer is slated to begin this month.
VinBioCare’s plant will be located at the Hanoi-based Hoa Lac Hi-tech Park, which is built at an investment of over US$200 million.
Necessary devices will be flown to Vietnam by September and the installation inside the plant is forecast to complete by two months later.
On August 2, Vingroup announced that it has successfully purchased 500,000 bottles of Remdesivir, which received approval by the Food and Drug Administration of the US for the treatment of COVID-19.
The medicine will be presented to the Ministry of Health in August.
Source: NDO
















.jpg)





.jpeg)

.jpeg)


.jpeg)


