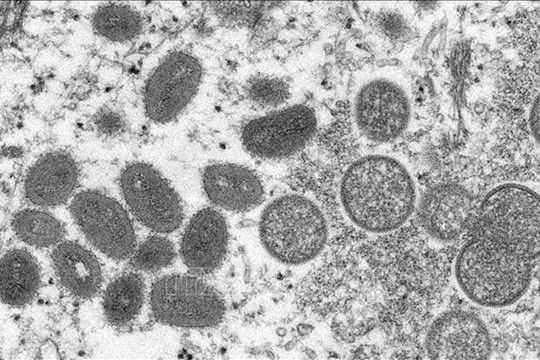
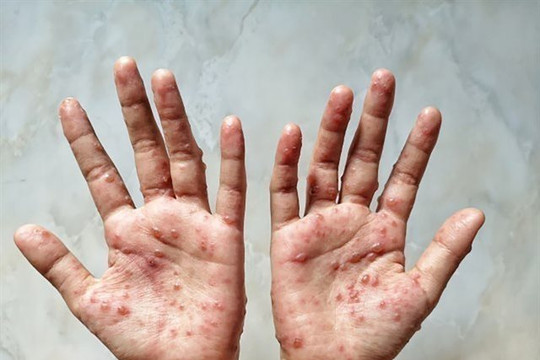
 |
On May 10, 25,057 people in 41 cities and provinces have been given the jabs.
The doses administered has reached 97 percent of the existing stock of 917,000
doses of AstraZeneca that Vietnam has received so far as part of the purchase of
30 million doses from the company and another 30 million from the global vaccine
sharing scheme COVAX Facility that is expected to be delivered in different
batches within 2021 and early 2022.
The first in line are mostly frontline workers – medical staff directly involved
with the treatment of COVID-19 patient; medical staff performing tasks such as
collecting patient samples, testing and tracing; members of grassroots
anti-COVID-19 groups; members of COVID-19 steering committees at local and
central levels; and police and military members.
Regarding last week's death of a medical worker in Vietnam who developed
anaphylactic shock after receiving the COVID-19 shot, health authorities have
stressed that the reaction rate is still too small to forego the critical
protection that the vaccines offer against COVID-19.
Pham Quang Thai, head of the northern region office of the NEPI, said all
vaccines, drugs, and even food – not just COVID-19 vaccines – could lead to
adverse reactions, but the rate is “very low” and if the vaccinated are
monitored and reactions are promptly dealt with then there would be few
problems.
“We are pained to hear about the incident. It was very rare, and we won’t pause
(the vaccination drive) here but we should learn from the experience here to
prevent such regrettable incidents in the future,” Thai said.
Specifically, there must be increased readiness for the prevention of
anaphylaxis at all immunisation facilities, according to the doctor.
Adrenalin shots must be readily available so that they could be immediately
administered, instead of wasting critical time in preparing the shots, he said,
adding that training and supervision of follow-up post-vaccination monitoring
should also be strengthened to ensure that medical staff are ready in all
situations.
Given the currently limited supply to Vietnam, especially as the COVID-19
situation is showing complicated developments, the health ministry has met with
a World Health Organization (WHO) representative to facilitate the negotiations
on transferring of mRNA technology – currently the cutting-edge technology used
in highly effective COVID-19 vaccines like Pfizer-BioNTech and Moderna (while
AstraZeneca used vector-based technology) – so that Vietnam can start producing
the vaccines right within the country.
The health ministry is awaiting the results of the second phase of the human
trials of Nanogen, Vietnam’s front runner in the race for a homegrown COVID-19
vaccine, and depending on the safety assessment, could start administering the
vaccine to the population as the phase 3 trials commence.
Source: VNA
















.jpg)





.jpeg)

.jpeg)


.jpeg)


