 |
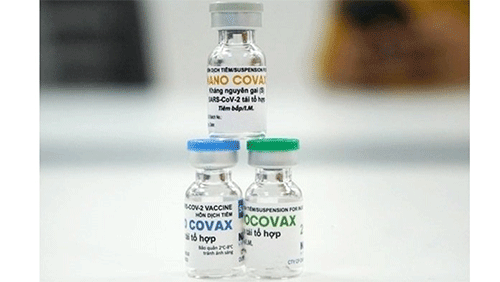
| The domestically-developed vaccine fully meets all safety and immunogenicity requirements |
Nano Covax has been developed by the Nanogen Pharmaceutical Biotechnology JSC based on recombinant DNA/protein technology since May 2020.
Currently, all dossiers and data have been transferred to the Advisory Council for issuance of circulation registration certificates of drugs and medicinal ingredients under the MoH for consideration and grant of conditional emergency licenses for Nano Covax vaccine.
Previously, the National Ethics Committee in Biomedical Research met from August 20-22 to evaluate the clinical trials of phase 3a Nano Covax vaccine, as well as evaluate the safety and immunogenicity of the vaccine.
According to a report at the meeting, the domestically-developed vaccine fully meets all safety and immunogenicity requirements.
Specifically, the ability to neutralise live virus of Nano Covax vaccine the 42nd day (i.e. 14 days after the second shot) is 96.5%.
Up to now, Nano Covax is Vietnam’s first COVID-19 vaccine to have gone through three stages of clinical trials. In the third phase, a total of more than 13,000 volunteers who have received 2 injections (1,004 people in phase 3a, and 12,000 people n phase 3b).
The Military Medical Academy and the Pasteur Institute in Ho Chi Minh City have been the two units conducting the trial, in which phase 3b was deployed in four provinces and cities including Hanoi, Hung Yen, Long An and Tien Giang.
Nanogen’s capacity is currently at 8-10 million doses per month and can be upgraded to 20-25 million per month.
Source: NDO
















.jpg)





.jpeg)

.jpeg)


.jpeg)


